Health policies and data
Performance-based managed entry agreements for new medicines
|
Managed entry agreements (MEAs) are arrangements between firms and healthcare payers that allow for coverage of new medicines while managing uncertainty around their financial impact or performance. Financial agreements are used in at least two-thirds of OECD countries and EU member states. Many of these countries also use performance-based agreements, which make coverage, payments to firms or rebates paid by firms conditional on product performance, but these MEAs are less common. With support from the European Commission, the OECD explored the experience countries have had thus far with performance-based MEAs to identify good practices and possible ways to improve the use of these agreements in the future.
|
 |
SCOPE OF THE STUDY
- This study covers a broad scope of managed entry agreements (MEAs), which are defined as: “Arrangements between a manufacturer and payer/provider that enable access to (coverage/reimbursement of) a health technology subject to specified conditions. These arrangements can use a variety of mechanisms to address uncertainty about the performance of technologies or to manage the adoption of technologies in order to maximize their effective use, or limit their budget impact.” (Klemp, Frønsdal and Facey 2011, p.79).
- The focus of the study is on performance-based MEAs, which make coverage, payments to firms or rebates paid by firms conditional on product performance. To allow for international comparability of the types of MEAs used across OECD countries and EU member states, the study uses the following 3-level taxonomy.
A taxonomy of Managed Entry Agreements
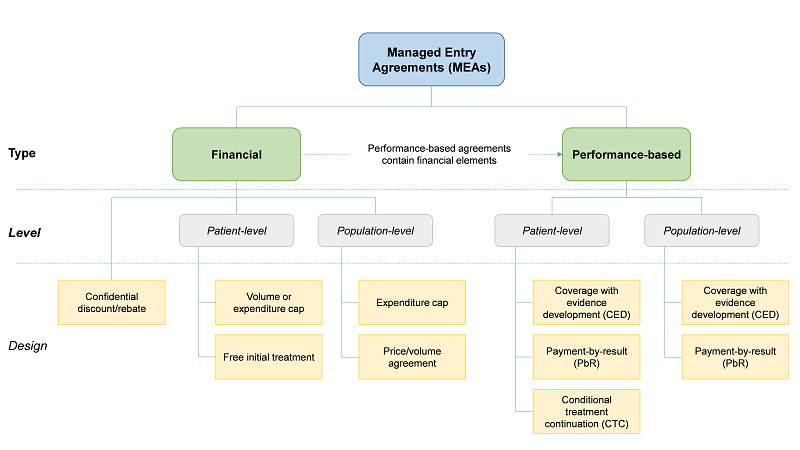
Note: This taxonomy is only based on how agreements are structured. All types of agreements above can exist not only between firms and healthcare payers but also between firms and other types of entities that constitute a health system, including government departments or national authorities responsible for coverage or pricing decisions and/or health technology assessment (HTA), regional health authorities, healthcare providers, etc. Especially for products used in the hospital inpatient sector, MEAs may be in place between firms and hospitals.
Source: Study Authors based on Carlson (2010), Ferrario and Kanavos (2013) and Gerkens et al. (2017).
KEY FINDINGS
It is difficult to assess to what extent performance-based MEAs have so far been successful. Few countries have formally evaluated their experience. Confidentiality of agreements continues to be a barrier to independent evaluation and little evidence is public. However, information available from expert interviews and from prior studies indicates that coverage with evidence development (CED) agreements have so far had a poor track record of reducing uncertainty around the performance of medicines. Payment-by-result (PbR) agreements continue to be used quite widely, but they do not always generate evidence on product performance because data used for triggering payments are not always aggregated and analysed. The administrative burden of collecting and analysing data on the performance of the medicines can also make them costly to execute.
Despite the lack of evidence, experience with performance-based agreements so far points to a number of good practices. These span four main themes:
- Defining a strategy to guide the use of performance-based MEAs and ensuring that they are used only where the benefit of additional evidence on product performance outweighs the cost of negotiating and executing MEAs;
- Clearly identifying uncertainties in each coverage decision and designing performance-based MEAs to ensure that data sources and research designs are appropriate to address the uncertainties at hand;
- Implementing a governance framework that ensures transparency of process and allows payers to act upon the additional evidence generated as a result of MEAs in accordance with that evidence, including exit from MEAs and potential withdrawal of temporary coverage; and
- Ensuring a minimum level of transparency of content, limiting confidentiality to those parts of MEAs that may be commercially sensitive (in particular prices).
HEALTHCARE PAYERS COULD GREATLY BENEFIT FROM INTERNATIONAL SHARING OF INFORMATION ON PERFORMANCE-BASED MANAGED ENTRY AGREEMENTS
While healthcare payers could greatly benefit from international sharing of information on performance-based MEAs, little information is currently shared or published. This is the case despite interest across countries in accessing information on the products for which MEAs are in place, on the design of MEAs and on their results. The figure below shows the number of countries in which there is interest in accessing different types of information related to performance-based MEAs vs. the number of countries in which such information is not confidential (i.e. published, or not published but not confidential and available upon request). There is interest in all countries in information on the existence of MEAs and on how product performance is measured. However, in particular information on how performance of products is measured under MEAs and the results of MEAs are often kept confidential. The potential non-disclosure of results of clinical studies conducted under performance-based MEAs raises ethical concerns as available information on the effectiveness of medicines could be withheld from the public. Greater sharing of information would benefit payers, for example, by reducing duplication of effort between countries, by allowing payers to learn from experience gained elsewhere to inform their negotiations with firms, and by reducing uncertainty that results from small patient samples (e.g. in the case of rare diseases). In addition, greater transparency of relevant information on the performance of products would also be useful for other stakeholders with legitimate interests and the general public.
Confidentiality of information on performance-based MEAs versus interest in such information
Number of countries in which information is not confidential and in which there is interest in such information from other countries, based on interviews with experts from 12 OECD countries(1) that use performance-based MEAs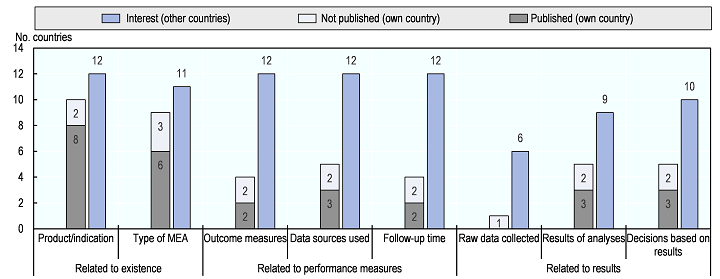
Notes: MEA… managed entry agreement.
- Published: information is readily available in the public domain (e.g. on the internet).
- Not published: information is not available in the public domain, but is not confidential and may be shared with 3rd parties upon request.
1. Australia, Belgium, Czech Republic, Estonia, France, Hungary, Italy, Korea, Lithuania, Netherlands, Sweden, United Kingdom (England only)
- Information for Australia refers to ad-hoc agreements only. Information for England refers to Managed Access Agreements only (31 agreements under the Cancer Drugs Fund and 4 for other disease areas reviewed by the OECD), which are publically available; not to other Patient Access Schemes.
Source: Study authors based on OECD expert interviews.
FURTHER READING
- Learn more about our work on Pharmaceuticals
- Addressing the challenges of access to medicines
CONTACT US
 Follow us on Twitter via @OECD_Social
Follow us on Twitter via @OECD_Social
Related Documents