Health policies and data
Using routinely collected data to inform pharmaceutical policies
|
Health systems routinely collect vast amounts of patient-level data. These data offer increasing opportunities to distil evidence from clinical practice, and to assess and monitor the effectiveness and safety, benefits and the costs of healthcare interventions. In many OECD and EU member countries, the breadth and volume of routinely collected electronic patient-level data are reaching a crescendo, but their capacity to generate and use evidence to inform health policies varies considerably. With support from the European Commission, the OECD explored countries' routine collection of data on prescribed and dispensed medicines to identify best practices, and to assess the potential impact on health and pharmaceutical policy.
 |
|
KEY FINDINGS
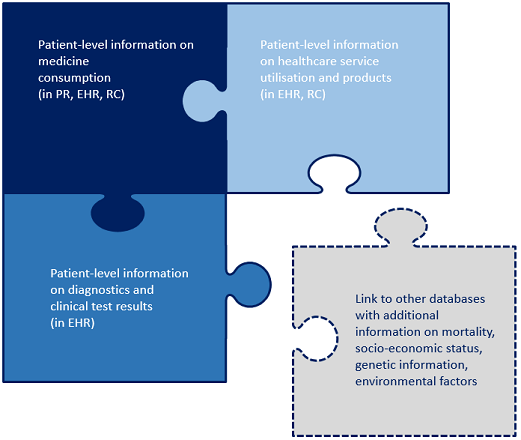
Routinely collected data on prescribed and dispensed medicines illuminate a key component of patients' interactions with the healthcare system. The data can be extracted from pharmacy records, personal health records, reimbursement claims and billing information. The richest data sources extend beyond prescribing and dispensing to information on utilisation of health services and outcomes of treatment. These datasets either capture this information directly, or can be dynamically linked with other health and healthcare datasets containing additional information.
- Download Using routinely collected data to inform pharmaceutical policies: Analytical Report
- Download the Factsheet to access the findings of the report in a nutshell
Notes: PR: Pharmacy records, EHR: Electronic Health Records, RCs: Reimbursement claims and billing information.
Source: Authors.
- Most countries routinely collect data on prescribed and dispensed medicines and make them available in national-level databases. Several countries are also able to generate large datasets of information related to patients' healthcare consumption, diagnoses and causes of death. However, healthcare systems in OECD and EU member countries vary in their technical capacity to harness the data for research or for the management of medicines in view of promoting evidence-based health policies.
- Access to routinely collected data is largely dependent on the mechanism and structure of healthcare coverage and the approach to the management of pharmaceuticals. About half the responding countries reported that routine databases were only accessible to government agencies and data custodians. In most of these, healthcare payers, public institutions involved in pharmaceutical decision making processes, and research institutions are the stakeholders most frequently granted access. In some countries (e.g. France, Netherlands, United Kingdom), data are also accessible to other stakeholders, e.g. healthcare providers and pharmaceutical sector operators.
- Although vast amounts of data are collected routinely, they are not used systematically to inform pharmaceutical and healthcare policies. Also, many countries report a potential data gap when it comes to monitoring the use of pharmaceuticals in hospital settings. As a result, not only is getting a complete picture of how routinely collected data are utilised complex, analysing the impact these data have on pharmaceutical policy development is even more challenging.
- Drawing on responses from the 2018 OECD Survey on Routinely Collected Data, together with a review of the literature and a series of expert interviews, this report describes how healthcare systems (countries) currently use routinely collected data to guide the management of pharmaceuticals. While the data are most often used to monitor national spending and medicines consumption, some countries consider the data to varying degrees in health technology assessment and reimbursement decision making, and in some cases regulatory agencies use them to inform post-marketing assessments.
SEVERAL COUNTRIES CONSIDER ROUTINELY COLLECTED DATA IN ASSESSMENTS AND DECISION-MAKING PROCESSES
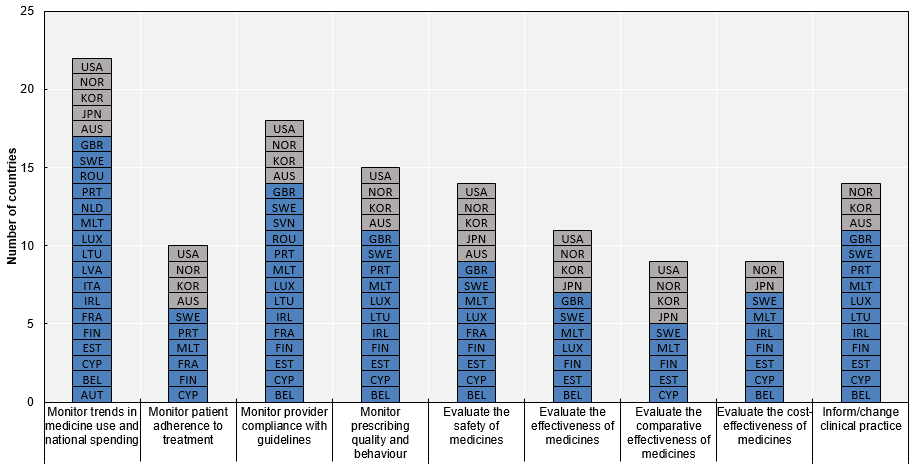
- Routinely collected data are primarily used to monitor medicine consumption and pharmaceutical spending, and to assess safety and providers' compliance with guidelines. About half the responding countries use the data to inform comparative effectiveness and cost-effectiveness analyses, while some countries also use them to conduct broader research on medicines. For example, the Clinical Practice Research Datalink is a particularly rich source of routinely collected data in the United Kingdom and has informed pharmaco-epidemiological research for more than 30 years. Also routine databases in Finland, France, Norway, Sweden are particularly rich in terms of population coverage and applicability to research.
Notes:
- Czech Republic: National Registry of Reimbursed Health Services operational since January 2018; Israel and Slovenia: missing information; Italy: response referring to AIFA monitoring registries; United States: the use of data depends on the specific data collection. N=25.
Note by Turkey: The information in this document with reference to “Cyprus” relates to the southern part of the Island. There is no single authority representing both Turkish and Greek Cypriot people on the Island. Turkey recognises the Turkish Republic of Northern Cyprus (TRNC). Until a lasting and equitable solution is found within the context of the United Nations, Turkey shall preserve its position concerning the “Cyprus issue”.
Note by all the European Union Member States of the OECD and the European Union: The Republic of Cyprus is recognised by all members of the United Nations with the exception of Turkey. The information in this document relates to the area under the effective control of the Government of the Republic of Cyprus.
Source: 2018 OECD Survey on Routinely Collected Data.
FURTHER READING
- Learn more about our work on Pharmaceuticals
- Addressing the challenges of access to medicines
CONTACT US
 Follow us on Twitter via @OECD_Social
Follow us on Twitter via @OECD_Social
Related Documents