Health policies and data
Value in Pharmaceutical Pricing
Countries are increasingly seeking to achieve better value for money in pharmaceutical spending, while keeping incentives to innovate. In this context, “value-based pricing” has gained momentum over the last years. According to this principle of “value-based pricing”, prices of pharmaceuticals should reflect their “value” to the society, as assessed against a range of criteria.
The Working Paper Value in Pharmaceutical Pricing released in July 2013 sheds light on the extent to which “value” is considered in pricing and/or coverage decisions in 14 OECD member countries (Australia, Belgium, Canada, Denmark, France, Germany, Italy, Japan, Korea, the Netherlands, Norway, Sweden, and the United Kingdom – England and Scotland). It describes methods used by countries to assess the therapeutic benefits of new products, as well as approaches for economic evaluation, where relevant. It illustrates how countries consider innovation, wider social benefits, severity or rarity of disease in their decisions, as well as the role of product-specific agreements. A sample of 12 products brought to the market between 2005 and 2011 is used to better illustrate how value is assessed and what is its impact on pricing and/or reimbursement decisions.
Main results and conclusions
The Working Paper is complemented by country profiles describing in details the pharmaceutical systems including decision-making processes for regulatory approval, reimbursement and pricing; assessment guidelines; institution and stakeholders involved and specific policies for new high cost drugs, when available.
- Value in Pharmaceutical Pricing, Health Working Paper No. 63 (July 2013)
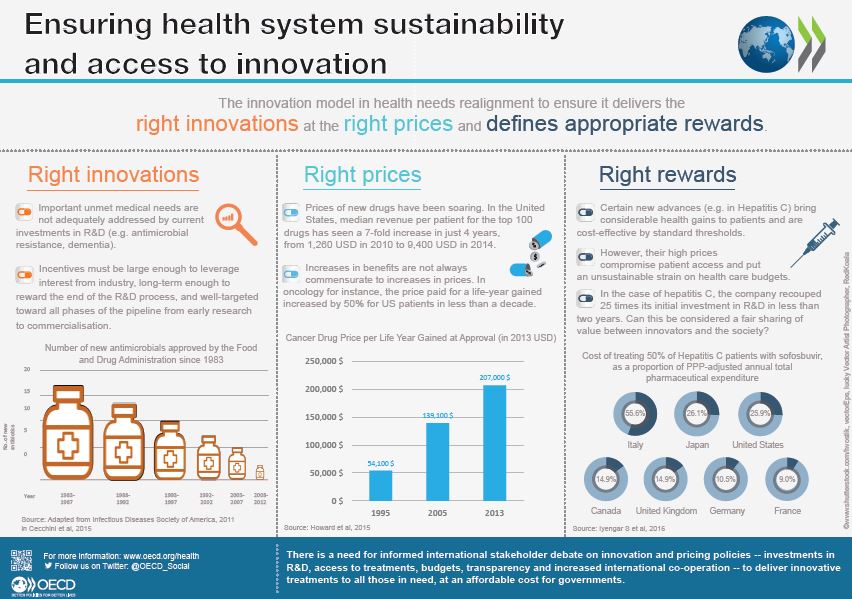
Download the infographics (September 2016)
Country profiles
 |
Australia |
 |
Canada |
Further reading
- Pharmaceuticals
- The OECD Health Division's Valérie Paris and Annalisa Belloni have contributed to a WHO-Europe report "Access to new medicines in Europe: technical review of policy initiatives and opportunities for collaboration and research", released in March 2015.
- Pharmaceutical Pricing Policy project
- OECD Review of Public Procurement of the Mexican Institute of Social Security
- G7 Health Ministerial meeting
CONTACT US
Ms Valérie Paris: valerie.paris@oecd.org
 Follow us on Twitter via @OECD_Social
Follow us on Twitter via @OECD_Social
Related Documents